Class 11th science chemistry (States Of Matter )
The intermolecular forces run between the particles of matter. There exists a pure electrostatic force between two ions which are oppositely charged. The intermolecular forces are different from these pure electrostatic forces. Determining the state of matter is done by the competition between intermolecular interactions and thermal energy. Various properties of matter in bulk such as a change in state, characteristics of liquids and solids, gases behavior depends on two factors:
- The interaction type between them
- The energy of constituent particles
The change of state does not affect the chemical properties of a substance, but the change in physical state affects the reactivity.
Boyle’s Law
Charles’s Law states that the volume of a given mass of gas is directly proportional to the absolute temperature at constant pressure. It is expressed as PV = k where P is pressure, V is volume and k is constant. This law is also referred to as the Boyle-Mariotte law or Mariotte’s law.
Charles’ Law
Charles’s Law states that the volume of a given mass of gas is inversely proportional to the absolute pressure at a constant temperature. It is expressed as V/T = k where V is the volume of gas, T is the temperature of the gas, and k is constant. The temperature is measured in Kelvin scale. This law is also termed as the law of volumes.
Few Important Questions
- Given: Quantity of gas = 4.0 mol occupies 5 dm3, Pressure = 3.32 bar, R = 0.083 bar dm3 K-1 mol-1.
- Calculate the molar mass of phosphorous with the following details:
The quantity phosphorus vapor is 34.05 mL and weight is 0.0625 g. At 0.1 bar pressure and 546 °C temperature.
- What is the physical importance of van der Waals parameters? Explain.
In 1662, Robert Boyle experimentally found a relationship between volume and pressure (Boyle’s law). He studied the relationship between volume and pressure with the help of air and mercury at room temperature. In his experiment, he used mercury and a simple U shaped tube.
He started applying pressure, by putting more mercury in the open limb. The volume enclosed by air is the space above mercury in the shorter limb. This volume decreases when we put some more mercury. He observed that volume decreases when pressure increases. After this experiment he concluded a relationship between pressure and volume which is also known as Boyle’s law. It is one of the fundamental gas laws in chemistry.
Know more about boy
“At a given temperature, the volume of a fixed amount of gas is inversely proportional to the pressure provided”.
Mathematically it can be stated as:
Or, V = k1p or PV =k (k is the constant of proportionality)
- PV = Constant at fixed temperature
Where, P- Pressure
V – Volume of the gas
‘k’ is the constant which is also known as Boyle’s constant. Value of k depends on the temperature of gas, amount of gas and on the units in which pressure and volume are expressed.
Suppose P1 and V1 are the initial pressure and volume of the given mass of gas. This gas now undergoes expansion at a fixed temperature and the pressure and volume changes to P2 and V2. Now, According to Boyle’s law:
P1V1 = P2V2 (at constant temperature)
This can be explained as: “At a fixed temperature, the product of volume and pressure for a fixed amount of mass of a gas is always constant”.
Know more about Boyle's law formula.
Graphical verification of Boyle’s law:
Fig.1 represents the equation PV = k.
In this, each curve shows pressure and volume relationship at a constant temperature and is called as an Isotherm.
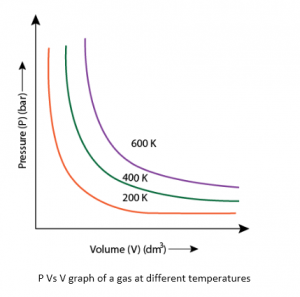
P Vs V graph of a gas at different temperatures
Fig.2 is a graphical presentation between P and 1/V.
Usually the graph shows a straight line, but at high pressure gases do not follow the Boyle’s law and straight line is not obtained in the graph.
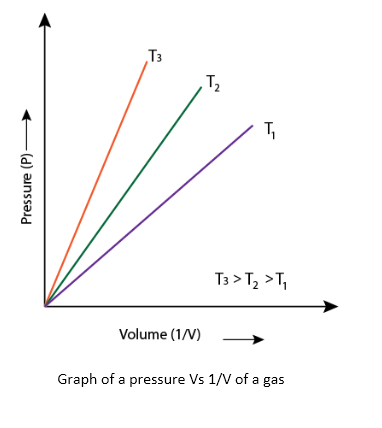
In 1787, the French scientist Jacques Charles discovered that volume of a gas varies when we change its temperature, keeping the pressure constant. Later, in 1802, Joseph Gay-Lussac modified the concept given by Charles and generalized it as Charles’s law. Gases obey Charles law at a very high temperature and low pressure.
It can be stated as:
“The volume of a fixed mass of a gas decreases on cooling it and increases by increasing the temperature. For one degree rise in temperature, the volume of the gas increases by 1273
Then, Vt Vo+t273.15Vo
We will now assign a new scale for temperature where the temperature in Celsius is given as t = T -273.15 and 0˚C can be given as To = 273.15. This new scale of temperature (T) is known as the Kelvin temperature scale or Absolute temperature scale. Degree sign is not written when a temperature is written in Kelvin scale. It is also known as the thermodynamic scale of temperature and it is commonly used in all scientific purposes. Thus, when we need to write temperature in Kelvin scale we add 273 to the temperature in Celsius.
Let us assume Tt = 273.15 + t
To = 273.15
Then equation (iii) can be written as
Or, (VtVo) (TtTo)
In general, we can write it as
Or, (V1T1) (V2T2)
⇒VT = constant = k2 .
Hence, V k2T
The value of k2 depends on the pressure of the gas, its amount and also on the unit of volume V.
Know more about Charle's law calculator.
Graphical representation:
At a fixed pressure, when the volume is varied, the volume-temperature relationship traces a straight line on the graph and on moving towards zero volume all lines intersect at a point on the temperature axis which is -273.15˚C. Each line in the graph of volume Vs temperature is known as isobar (Since pressure is constant). The least hypothetical temperature of -273˚C at which a gas will have zero volume is called as absolute zero.

Comments
Post a Comment