Class 11th science chemistry (thermodynamics)
Thermodynamics deals with the change of energy in physical and chemical processes. It allows us to study these changes quantitatively as well as to make effective predictions. Therefore, we divide the universe as surrounding and system. Physical or chemical processes lead to progression or immersion of heat part of it may be converted to work. According to the first law of thermodynamics, these quantities are related as ∆U = q + w where q is heat and w is work. Change in internal energy is a state function and depends on only the initial and final states. On the other hand, w and q are not state functions. The change in temperature due to the transfer of heat one system to another system can be measured.
- Heat evolved or absorbed is expressed as q = C∆T.
- In case of expansion of gas, work is measured as w = –pex∆V
Enthalpy Change
Enthalpy change is defined as the change in energy of a system at constant pressure. It is denoted as ΔH. Enthalpy is a state function. Enthalpy change, ∆H = ∆U + ∆ngRT, can be calculated directly from the change in heat at constant pressure, ∆H = qp
Hess’s law
Hess’s law states that the standard reaction enthalpy for overall reaction taking place in several steps will be equal to the sum of standard enthalpies of intermediate reaction steps by assuming that each step occurs at the same temperature.
Hess’s Law- The law for constant heat summation was derived in the year 1840, a Swiss-born Russian chemist and physician, Germain Hess, derived a relationship in thermochemistry for calculating the standard reaction enthalpy for multistep reactions. It basically exploits the properties of state functions, that is the value of state functions do not depend on the path taken for formation or dissociation rather it depends only on state at the moment (temperature, pressure, volume, etc). As we know enthalpy is a state function, hence, it is independent of the path taken to reach the final state starting from the initial state. According to Hess’s law, “for a multistep reaction the standard reaction enthalpy is independent of the pathway or number of steps taken, rather it is the sum of standard enthalpies of intermediate reactions involved at the same temperature”.
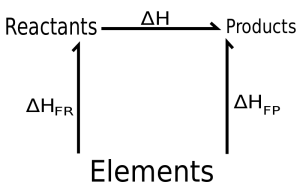
Application of Hess Law:
Let us look at some practical areas where Hess’s law is applied, for example, the formation of Sulphur Trioxide gas from Sulphur is a multistep reaction involving the formation of sulphur dioxide gas. Let us find out the standard reaction enthalpy for the formation of Sulphur Trioxide gas from Sulphur.
Step 1: Formation of Sulphur Dioxide gas
Step 2: Conversion of Sulphur Dioxide gas to Sulphur Trioxide gas
Standard reaction enthalpy as per Hess’s Law:
Net reaction:
Hence, in simple words we can state:
Determination of free energy and entropy:
As with enthalpy, Hess’s Law can be used to determine other state functions such as free energy and entropy, for example: Bordwell thermodynamic cycle which takes advantage of easily measured equilibriums and Redox potentials to determine experimentally inaccessible gibb's free energy values.
As entropy can be measured as an absolute value, hence in case of entropy there is no need to use entropy of formation.
Introduction
While dealing with a chemical reaction, the knowledge of enthalpy and standard enthalpy is very important. It helps in calculating the temperature and pressure required for any chemical reaction. It is also required for calculating the amount of heating and cooling necessary when the reaction is carried out commercially (at a large scale), for example, for the mass production of any component such as ammonia, calcium carbonate, oxygen etc. In this section, we will learn about the calculation of the change of enthalpy for a chemical reaction.
Enthalpy change
Enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. In any general chemical reaction, the reactants undergo chemical changes and combine to give products. It can be represented by the following equation:
For any such reaction, the change in enthalpy is represented as ΔrH and is termed as the reaction enthalpy. The reaction enthalpy is calculated by subtracting the sum of enthalpies of all the reactants from that of the products.
Mathematically,
ΔtH = Sum of enthalpies of the product – Sum of the enthalpies of the reactants.
Here, the constants ai and bi denotes the stoichiometric coefficients of the products and the reactants respectively for the balanced chemical reaction under consideration.
Standard enthalpy of reaction
We already know the enthalpy of any reaction depends on the physical conditions of the surrounding such as the temperature, pressure, etc. In order to specify the standard enthalpy of any reaction, it is calculated when all the components participating in the reaction i.e., the reactants and the products are in their standard form. Therefore the standard enthalpy of reaction is the enthalpy change that occurs in a system when a matter is transformed by a chemical reaction under standard conditions.
As per convention, the standard state for any substance at a specified temperature is its pure form at a pressure of 1 bar. Foran example, liquid ethanol at 298 K and 1 bar of pressure is said to be in its standard state in its pure form. It is important to note that the data for the standard state for a substance is taken at 298K. The standard enthalpy of a reaction is denoted as ΔrHs. At constant pressure, the heat of the reaction is exactly equal to the enthalpy change, of the reacting system.
Comments
Post a Comment