Class 11th science physics (kinetic theory)
What is Kinetic Theory of Gas?
The kinetic theory of gases explains the behavior of molecules, which should further explain the behavior of an ideal gas. Ideal Gas equation consists of the pressure (P), volume (V), and temperature (T) of gases at low temperature and the equation is: PV = nRT
Where n = number of moles in the gas R = gas constant having value 8.314JK−1mol−1
Now, any gas following this equation is termed as an ideal gas. Below are some certain assumptions that we consider for describing ideal gas behavior.
What are the Assumptions of Kinetic Theory of Gases?
- All gas molecules constantly move in random directions.
- The size of molecules is very less than the separation between the molecules
- The molecules of the sample do not exert any force on the walls of the container during the collision when the gas sample is contained.
- It has a very small time interval of collision between two molecules, and between a molecule and the wall.
- Collisions between molecules and wall and even between molecules are elastic in nature.
- Newton’s laws of motion can be seen in all the molecules in a certain gas sample.
- With due course of time, a gas sample comes to a steady state. The molecule’s distribution and the density of molecules do not depend on the position, distance and time.
What is law of equipartition of energy?
According to this law, when a system is in equilibrium provided the temperature is absolute, the total energy tends to be distributed evenly in the various energy modes of the absorption. Each translational and the rational degree of freedom represents one energy mode of absorption.
With the help of this law, we can determine the values of molar specific heat of gases.
In physics, an inelastic collision occurs, when the maximum amount of kinetic energy of a colliding objects/system is lost. The colliding particles stick together in a perfectly inelastic collision. In such cases, kinetic energy lost is used in bonding the two bodies together. Problems involving collisions are usually solved using conservation of momentum and energy.
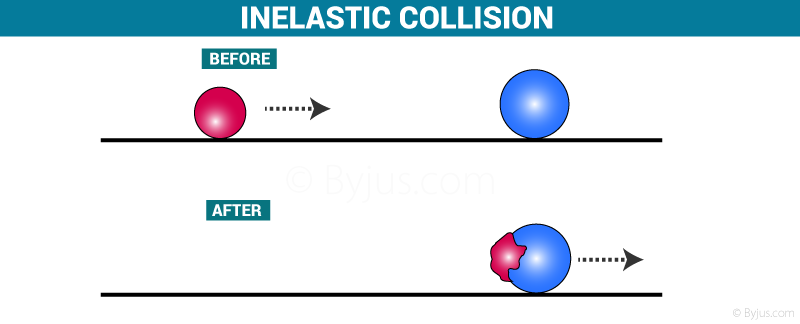
What is a collision? A collision is an event in which two or more objects exert forces on each other for a short interval of time. It is categorized into two types,
- Inelastic collision
- Elastic collision
Inelastic Collision Definition
An inelastic collision is such a type of collision that takes place between two objects in which some energy is lost. In the case of inelastic collision, momentum is conserved but the kinetic energy is not conserved. Most of the collisions in daily life are inelastic in nature.
Perfectly Inelastic Collision
The special case of inelastic collision is known as a perfectly inelastic collision. Here, after collision two objects stick together. Refer to the figure above. Example: when wet mudball is thrown against a wall mudball stick to the wall.
Inelastic Collision Formula
When two objects collide under inelastic condition. The final velocity with which object move is given by-
Where,
- V= Final velocity
- M1= mass of the first object in kgs
- M2= mas of the second object in kgs
- V1= initial velocity of the first object in m/s
- V2= initial velocity of the second object in m/s
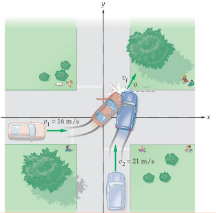
Inelastic Collision in Two Dimension
Inelastic collision in two dimensions, Conservation of momentum is separately applied separately along each axis. Because Momentum is a vector equation and there is one conservation of momentum equation per dimension. Similarly, there is only one conservation of energy equation.
Inelastic Collision Examples
Most of the collision we see in our day to day life falls under inelastic collision. Some of them are listed below.
Inelastic Collision Examples Real World
- The ball is dropped from a certain height and it is unable to rise to its original height.
- When soft mudball is thrown against the wall, will stick to the wall.
- The accident of two vehicles
- A car hitting a tree.
Inelastic Collision Kinetic Energy
In the case of inelastic collision, the kinetic energy is not conserved. The loss of kinetic energy is due to internal friction. It may turn into vibrational energy of the atoms, causing a heating effect, and the bodies are deformed.
Elastic Collision
Any collisions in which the collided objects get separated after the collision is known as an elastic collision. In case of elastic collision kinetic energy gets conserved. One must use both conservation of momentum and conservation of energy to find the motions of the objects later.
Some examples of elastic collisions are Ping-pong balls, billiards, etc.
Comments
Post a Comment