Class 11th science chemistry(structure of atom)
As discussed in the previous chapter, according to Dalton’s atomic theory, atoms are the building blocks of matter. In the year 1808, John Dalton proposed atomic theory saying that atom is an indivisible particle of matter. By the end of the nineteenth century, experiments proved that atoms comprise of three particles viz protons, neutrons, and electrons. Therefore, the discovery of sub-atomic particles gave rise to various atomic models to explain the structure of an atom.
Common terminologies used – Structure Of Atom
- Electrons – These subatomic particles orbit around the nucleus. They are negatively charged particles and found in definite energy levels orbiting the nucleus.
- Protons – Theses subatomic particles are found in the nucleus. They are positively charged particles and belong to the nucleons group.
- Neutrons – Theses subatomic particles are found in the nucleus. They are neutral particles and belong to the nucleons group.
- Rutherford model – It states that during a chemical reaction, the mass of the products and reactants will always be equal.
- Bohr model – The model states that electrons orbit stable around the nucleus in certain fixed circular orbits at a distinct distance from the nucleus. These orbits are related to specific energies and are also referred to as energy levels or energy shells.
- Heisenberg uncertainty principle – The Heisenberg Uncertainty Principle states that the simultaneous determination of the velocity and position of a particle is impossible.
- Shells – A pathway followed by the electron to move around the nucleus of the atom.
- Subshells – A pathway for the electron to move within a shell.
Bohr model of the atom was proposed by Neil Bohr in 1915. It came into existence with the modification of Rutherford’s model of an atom. Rutherford’s model introduced the nuclear model of an atom, in which he explained that a nucleus (positively charged) is surrounded by negatively charged electrons. Bohr modified this atomic structure model by explaining that electrons move in fixed orbital’s (shells) and not anywhere in between and he also explained that each orbit (shell) has a fixed energy level. Rutherford basically explained nucleus of an atom and Bohr modified that model into electrons and their energy levels.
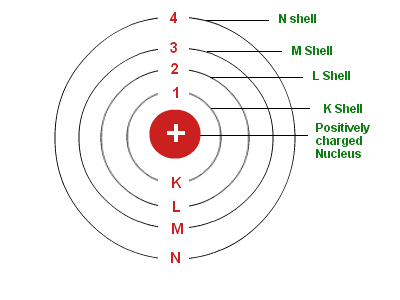
Bohr’s Model of an Atom
Bohr’s model consists of a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbits. Bohr found that an electron located away from the nucleus has more energy, and electrons close to the nucleus have less energy.
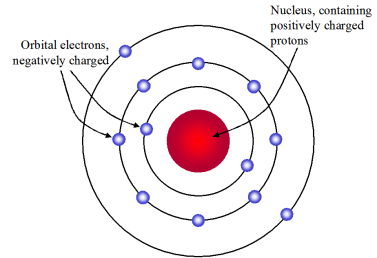
Rutherford was always curious in knowing about the arrangement of electrons in an atom. By performing an experiment using alpha particles and gold foil he came to some conclusions.

Rutherford’s Model
Experiments performed
Let us first learn something about the experiments he performed:
- A 1000 atoms thick gold foil was selected because he wanted as thin a layer as possible.
- Alpha particles are nothing but doubly charged helium ions. As its mass is 4u, the fast moving alpha particles have good amount of energy.
- He also expected that the alpha particles will be deflected as they are heavier than the protons. But what he observed was completely unexpected, he made the following observations:
- Most of the alpha particles passed straight through that gold foil.
- There was a deflection by small angle by some of the alpha particles.
- Very small amount of alpha particles rebounded.
Rutherford concluded the following points after the observation:
- As there was very less deflection of alpha particles so he concluded that most of the space was empty in an atom.
- He also concluded that positive charge contains very less amount of space in an atom as very few articles were deflected from their path.
- A very small amount of alpha particles deflected with an angle of 1800, which indicated that the mass of the atom and the positive charge was concentrated at a small volume in an atom.
From all these observations he calculated that the radius of the nucleus is around 105 times less than the radius of the atom.
The following final model was put by Rutherford after all the observations:
- The nucleus is at the centre and is positively charged and nearly all the mass of the nucleus resides in the nucleus.
- Around the nucleus, electrons revolve in a circular path.
- The size of the nucleus is very less as compared to the size of the atom.
Comments
Post a Comment