Class 11th science chemistry (hydrogen)
The lightest atom is on earth is hydrogen which has a single electron. In the year 1766, Henry Cavendish was the first to discover it. The three stable isotopes of hydrogen are protium, deuterium, and tritium. The radioactive isotope amongst these three is tritium. Hydrogen is one of the most abundant elements found in the universe. Even though it resembles halogen and alkali metals, it has unique properties and has a separate position in the periodic table.
Water-Gas Shift Reaction
On an industrial scale level, Dihydrogen is prepared from petrochemicals by water-gas shift reaction. It is got in the form of byproduct on the electrolysis of brine.
Bond Dissociation Enthalpy
Dihydrogen has the highest H-H bond dissociation enthalpy for a single bond between the two atoms. The H-H bond dissociation enthalpy equals to 435.88 kJ mol–1
Hydrides
Even though at room temperature, dihydrogen is inactive due to high negative dissociation enthalpy, it can combine with nearly all the elements to form hydrides in suitable conditions.
Hydride can be classified into three different categories:
- Saline or Ionic hydrides – Good reagent to prepare additional hydride compounds
- Molecular or Covalent hydrides – Important for daily life. Example H2O
- Non-stoichiometric or metallic hydrides – Used for ultra purification of dihydrogen
Compounds of hydrogen with less electronegative elements are known as Hydrides. In periodic table Hydrides formation is not seen from VA group elements hence it is known as hydride gap.
Hydrogen molecule reacts with many elements except the noble gases to form hydrides. An element Y when reacts with hydrogen forms YHx , for example, MgH2. Hydride is actually an anion of hydrogen.
Types of hydrides
On the basis of chemical bonding, hydrides are of following types.
Ionic or salt like hydrides
They are formed when hydrogen molecule reacts with highly electropositive s-block elements (Alkali Metals and Alkaline Earth Metals). In solid state, the ionic hydrides are crystalline, non-conducting and non-volatile. However, in a liquid state, they conduct electricity. Ionic hydrides on electrolysis liberate hydrogen gas at the anode.
Example of Ionic Hydrides: Nah, KH, CaH2, etc. These contain the H– ion.
Covalent hydrides
These are hard and formed by hydrogen and other similar electronegative elements like Si, C, etc. The most common examples are CH4 and NH3. The hydrogen compounds formed with non-metals are also called hydrides. They are covalent and volatile compounds.
Example of Covalent Hydrides: SiH4(silane)
Metallic hydrides
These are formed by transition metals. These are mostly non-stoichiometric, hard, high melting and boiling points.
Example of Metallic Hydrides: TiH.
They are formed when hydrogen molecule reacts with the d- and f-block elements. Metals of group 7, 8, and 9 do not form hydrides. They do conduct heat and electricity but not to the extent of their parent metals.
They are used as reducing agents in many chemical industries. Hydrides are highly significant in battery storage technologies such as nickel hydride batteries.
What are Isotopes?
Let us take an example of two things which have the same color, same physical appearance, such that you cannot distinguish between these two. But when you measure the weight of these two things then you find that it is different. You can relate the concepts of isotopes with this example.
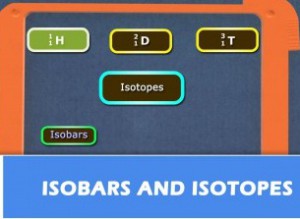
As we all know that atoms are made up of electrons, protons, and neutrons. The nucleus is made up of protons and neutrons and the electrons revolve around the nucleus. Atomic mass is the sum of some protons and the number of neutrons and atomic number is equal to the number of protons. In an element, the number of protons is always the same, but the number of neutrons keeps on changing.
Isotopes are the atoms in which the number of neutrons differs and the number of protons is the same. From the above definition of atomic mass and the atomic number, we can conclude that isotopes are those elements having a same atomic number and different mass number.
Let us know something about the isotopes of hydrogen: There are three isotopes of hydrogen and these are protium, deuterium, and tritium. All three of them have a same number of protons, but the numbers in neutrons differ. In protium the number of neutrons is zero, in deuterium, it is one and in tritium, the number of neutrons is two.
What are Isobars?
Isobars are those elements which differ in the chemical property but have same physical property. So, we can say that isobars are those elements which have a different atomic number but the same mass number. Their chemical property is different because there is the difference in the number of electrons. It has the same atomic mass but different atomic no. Because an additional number of neutrons compensates the difference in the number of nucleons.
The example of two Isotopes and Isobars is iron and nickel. Both have the same mass number which is 58 whereas the atomic number of iron is 26, and the atomic number of nickel is 28.
Comments
Post a Comment